Introduction
Pramipexole, a dopamine D2/D3 receptor agonist initially developed for Parkinson’s disease and restless legs syndrome, has shown emerging utility as an off-label treatment for bipolar depression. Its mechanism—preferential D3 stimulation—targets motivational and reward pathways implicated in anhedonia and psychomotor retardation, key features of bipolar depression unaddressed by traditional antidepressants. While pramipexole is not FDA-approved for mood disorders, a growing body of evidence, including randomized controlled trials (RCTs), open-label studies, and longitudinal observations, supports its efficacy and safety as an adjunctive treatment in bipolar I and II depression.
This review synthesizes clinical findings across controlled and observational studies to evaluate pramipexole’s therapeutic role, dose-response characteristics, tolerability, and risk of affective switch. We begin with a comparative overview of RCTs, followed by a focused side effect and safety profile, before detailing individual studies and meta-analyses.
Comparative Table of RCTs: Pramipexole in Bipolar Depression
| Study (Year) | Bipolar Type | N | Dose Range (mg/day) | Outcome Measure | Response Rate | Switch Rate | Notes |
|---|---|---|---|---|---|---|---|
| Goldberg et al. 2004 | I & II | 22 | ~0.25–1.7 | HDRS | 67% (vs 20%) | 1/12 (8%) | Adjunct to MS |
| Zarate et al. 2004 | II | 21 | ~0.25–2.25 | MADRS | 60% (vs 9%) | 1/10 (10%) | Adjunct to Li/VPA |
| PAX-BD 2023 | I & II | 39 | 0.25–2.5 | QIDS-SR16 | Trend only | ↑ in PPX arm | Underpowered |
| Hori et al. 2012 | II & MDD | 17 | Mean 1.6 | HDRS | 71% | 0 | Excellent tolerability |
Side Effect Profile Across Trials
| Study | Nausea | Fatigue | Somnolence | Mania/Hypomania | Dropout Rate |
| Goldberg 2004 | ✔ | ✔ | (not noted) | 1 case | Low |
| Zarate 2004 | ✔ | 1 case | Very Low | ||
| PAX-BD 2023 | ✔✔ | ✔✔ | ✔ | Higher vs placebo | Moderate |
| Fawcett 2016 | ✔✔✔ | ✔ | ✔✔ | 0 | Nausea-driven |
Dose-Dependent Response, Side Effects, and Switch Risk
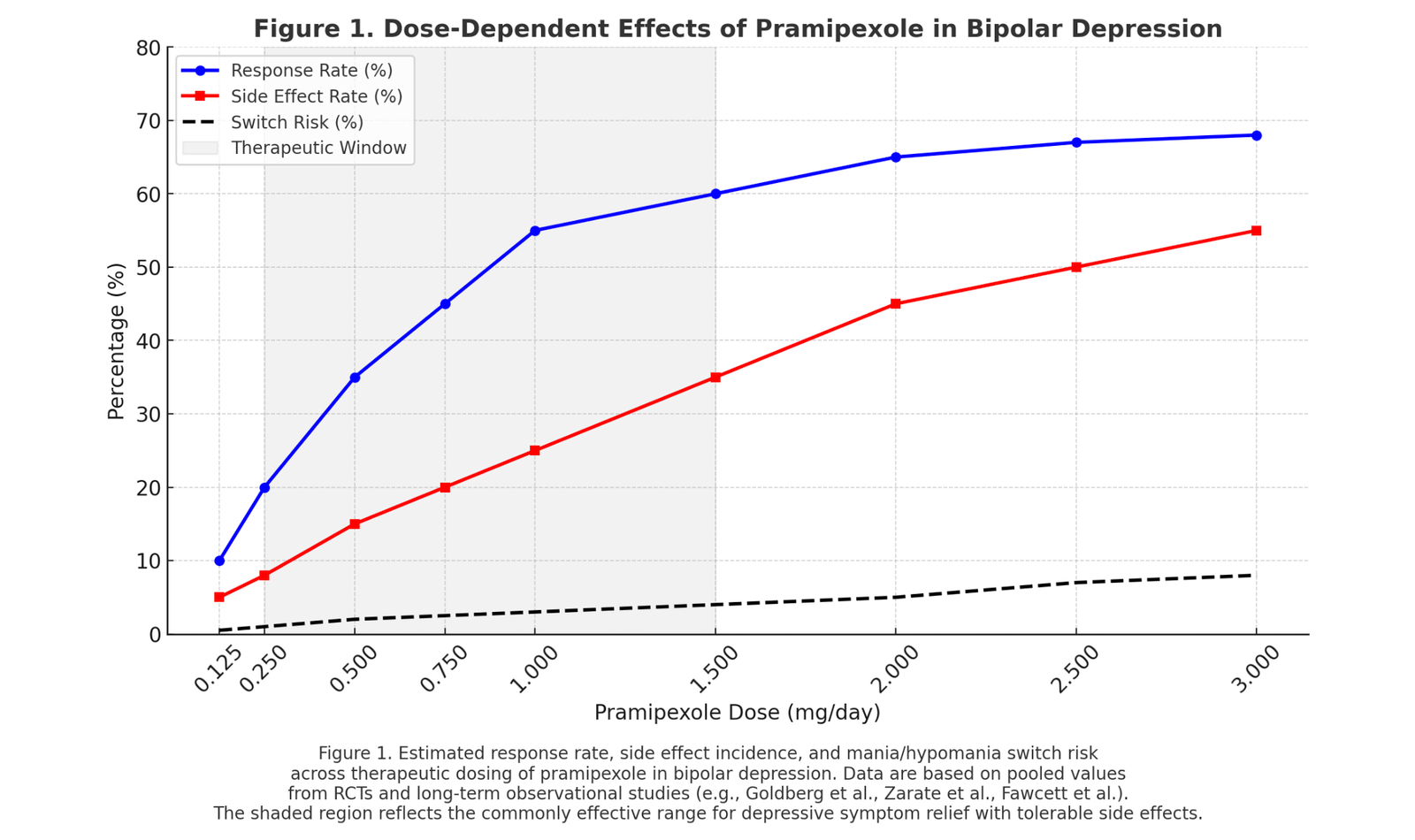
Figure 1 illustrates the clinical behavior of pramipexole across its dosing spectrum in bipolar depression. Response rates begin to rise meaningfully at 0.25 mg/day, increase steeply through the 0.75–1.5 mg/day range, and plateau near 2–2.5 mg/day. This range defines the therapeutic window, where efficacy is maximized with tolerable risk.
Side effect incidence—primarily nausea, fatigue, and somnolence—tracks upward with dose, but remains manageable below 2 mg/day in most patients.
Mania/hypomania switch risk remains low at therapeutic doses when pramipexole is used adjunctively with a mood stabilizer, with reported switch rates in RCTs comparable to placebo. Risk increases modestly above 2 mg/day, particularly in bipolar I patients or those with a history of affective instability.
This chart synthesizes pooled trends from randomized controlled trials (Goldberg et al., 2004; Zarate et al., 2004; PAX-BD, 2023) and observational series (Fawcett et al., 2016), guiding dose selection toward maximal benefit with minimal destabilization.
Following this foundation, we present detailed summaries of each clinical trial, including design, population, dosing, outcomes, and tolerability data, as well as meta-analytic interpretations and proposed clinical usage parameters.
Randomized Controlled Trials (RCTs)
Goldberg et al. (2004) – Adjunctive RCT in Treatment-Resistant Bipolar Depression
- Study Design/Year: 6-week preliminary double-blind RCT (2004) adding pramipexole vs. placebo to mood stabilizers.
- Population: 22 bipolar (I or II) depressed outpatients (non-psychotic, treatment-resistant). All patients remained on therapeutic mood stabilizers during the trial.
- Pramipexole Dose: Flexible titration (starting 0.125–0.25 mg) up to a mean maximum dose ~1.7 mg/day (SD 1.3) over 6 weeks.
- Comparators: Placebo plus continued mood stabilizer(s).
- Primary Outcome: Hamilton Depression Rating Scale (HDRS) response (≥50% score improvement). Pramipexole yielded a 67% response rate vs. 20% on placebo. Mean HDRS improvement was greater with pramipexole (−48% from baseline) than placebo (−21%). Global severity also improved more on pramipexole.
- Side Effects/Dropouts: Tolerability was generally good. 83% of pramipexole patients vs. 60% on placebo completed the trial. Nausea and fatigue were noted as common pramipexole side effects in later analyses, but no specific side effect rates were detailed in the abstract. No patient stopped due to adverse events except one case of hypomania.
- Mania/Hypomania Risk: 1 of 12 pramipexole patients (8%) developed hypomania and was withdrawn. No manic symptoms were reported in the placebo group during the 6 weeks.
Zarate et al. (2004) – RCT in Bipolar II Depression (Proof-of-Concept)
- Study Design/Year: 6-week double-blind placebo-controlled trial (2004) of adjunctive pramipexole in bipolar II depression.
- Population: 21 patients with DSM-IV bipolar II disorder, depressive phase, maintained on lithium or valproate mood stabilizers.
- Pramipexole Dose: Flexible dosing up to ~1.5–2.25 mg/day (exact titration not in abstract). Mean final dose was not stated in abstract, but similar studies achieved ~1.5 mg/day. Treatment duration was 6 weeks.
- Comparators: Placebo plus continued lithium/valproate.
- Primary Outcome: Montgomery–Åsberg Depression Rating Scale (MADRS) change. Pramipexole showed significantly greater antidepressant effect than placebo. Response rate (≥50% MADRS reduction) was 60% on pramipexole vs. 9% on placebo (p=0.02). MADRS scores improved markedly more with pramipexole, confirming a significant drug effect.
- Side Effects/Dropouts: All but one patient in each arm completed the 6 weeks, indicating good short-term tolerability. The abstract notes no serious adverse events; nausea and somnolence were likely but not detailed.
- Mania/Hypomania Risk: 1 patient on pramipexole (10%) developed emergent hypomanic symptoms, versus 2 patients on placebo developing hypomania. No full manic episodes occurred. Authors noted pramipexole’s antidepressant benefit without a high switch rate in this short trial.
PAX-BD Trial (2023) – RCT in Treatment-Resistant Bipolar Depression
***The PAX-BD study was unfortunately closed down early, prior to recruitment of a full sample of participants by the funder due to issues with the rate of recruitment. As a result, while there were some positive findings, conclusions from the study are somewhat limited.
- Study Design/Year: 12-week multi-center randomized placebo-controlled trial (PAX-BD, results 2023) of pramipexole added to mood stabilizers, with a 36–48 week extension for long-term outcomes.
- Population: 39 patients with treatment-resistant bipolar I or II depression (TRBD) under specialist care. (Trial enrollment was halted early at 39, limiting power). All participants were maintained on standard mood stabilizers throughout.
- Pramipexole Dose: Titrated from 0.25 mg up to 2.5 mg once daily (flexibly dosed by week 6 based on tolerability/efficacy). Mean achieved dose ~2 mg.
- Comparators: Placebo added to ongoing mood stabilizer therapy.
- Primary Outcome: QIDS-SR16 depression scale change at 12 weeks. Pramipexole showed a greater reduction in depressive symptoms than placebo, but the difference was not statistically significant in this underpowered sample. By 12 weeks, both groups improved; the pramipexole advantage did not meet significance (trend favoring pramipexole).
- Secondary Outcomes: At 36–48 weeks, pramipexole-treated patients showed some statistically significant benefits in mood, everyday functioning, and quality of life compared to placebo. Response and remission rates by 48 weeks favored pramipexole on some measures (data not in abstract). A cost-effectiveness analysis also trended positive for pramipexole.
- Side Effects/Dropouts: Pramipexole was generally well tolerated in this trial. However, more pramipexole patients experienced nausea, fatigue, and dizziness (consistent with known side effects) than placebo (exact rates not reported). The overall dropout rate was not explicitly reported in the summary.
- Mania/Hypomania Risk: Investigators noted an increased risk of elevated mood symptoms with pramipexole: more pramipexole patients had treatment-emergent hypomania/mania than placebo. (The abstract suggests pramipexole was associated with a higher incidence of mood switching, though exact numbers are not given due to the small N.) This signal aligns with the need for mood stabilizer co-therapy and careful monitoring.
Open-Label and Observational Studies
Sporn et al. (2000) – Retrospective Chart Review (Adjunctive Pramipexole)
- Study Type/Year: Retrospective chart review (2000) evaluating pramipexole augmentation in refractory depression.
- Population: 32 outpatients with treatment-resistant depression (12 bipolar depression, 20 unipolar depression) in a university hospital setting.
- Pramipexole Dose: Mean dose ~0.70 mg/day; treatment duration averaged 24 weeks. Doses were titrated gradually; many patients remained on ~1 mg or less (reflecting cautious early use).
- Outcomes: Clinical Global Impression–Improvement (CGI-I) ratings indicated a moderate-to-marked improvement in 50% of bipolar patients and 40% of unipolar patients. In total, 14 of 32 (44%) were classified as responders to pramipexole augmentation. These uncontrolled pilot results suggested appreciable antidepressant effects in both bipolar and unipolar cases.
- Side Effects/Dropouts: 8 patients (25%) discontinued pramipexole for lack of effect, and 4 (12%) discontinued due to side effects. Common adverse effects included nausea and sedation (typical of dopamine agonists). Overall, pramipexole was deemed “effective and safe” in this naturalistic sample.
- Mania/Hypomania Risk: One patient (~3%) had a transient hypomanic episode during pramipexole therapy , which resolved after stopping the drug. No full manias were reported, and no negative interactions with mood stabilizers were observed.
Perugi et al. (2001) – Open Case Series in Bipolar II Depression
- Study Type/Year: Open-label case series (chart review, 2001) of adjunctive dopamine agonists in bipolar II depression.
- Population: 18 bipolar II depressed inpatients (DSM-III-R Bipolar NOS, essentially bipolar II) with antidepressant treatment resistance. All were on antidepressants + mood stabilizers without adequate response for ≥8 weeks.
- Intervention: Pramipexole (PPX) or ropinirole (RPN) was added to ongoing treatments. 10 patients received pramipexole and 8 received ropinirole in this series.
- Dosing: Pramipexole was titrated to a mean final dose of ~1.23 mg/day (range 0.75–1.5 mg), while ropinirole averaged ~2.97 mg/day (range 1.5–5 mg). Mean augmentation duration was ~17.6 weeks.
- Outcomes: Using final CGI-I scores, 8 of 18 patients (44.4%) were responders (4 on pramipexole, 4 on ropinirole). Five patients (28%) achieved marked improvement (CGI-I=1) and three had moderate improvement (CGI-I=2). An additional 5 patients had transient improvements that were not sustained by endpoint . Overall depression severity (CGI-S) dropped significantly from baseline (mean 5.3 down to 3.9).
- Side Effects/Dropouts: Dopamine agonists were generally well tolerated. Only one patient (receiving pramipexole) had to discontinue due to side effects – this patient experienced worsening agitation/irritability and nausea early in treatment. No serious adverse interactions with co-medications occurred.
- Mania/Hypomania Risk: No instances of mania or hypomania were reported in this bipolar II cohort during the trial. The only patient who “became worse” did so via increased irritability (not frank mania). Authors concluded pramipexole and ropinirole “appear to be well tolerated and potentially useful” adjuncts in bipolar II depression.
Lattanzi et al. (2002) – 16-Week Open Trial of Adjunctive Pramipexole
- Study Type/Year: Prospective open-label trial (2002) over 16 weeks, assessing pramipexole added to antidepressants.
- Population: 37 inpatients with DSM-IV major depressive episodes resistant to treatment (failure of prior antidepressants). This included 21 bipolar (I or II) and 16 unipolar depressed patients. All continued their existing tricyclic or SSRI antidepressant during pramipexole augmentation.
- Pramipexole Dose: Titrated from 0.375 mg to 1.0 mg/day max over several weeks. The mean maximal dose achieved was 0.95 mg/day , reflecting a low-dose strategy.
- Outcomes: Depression severity improved significantly. Mean MADRS score fell from 33.3 at baseline to 13.9 at week 16 (p<0.001). CGI-Severity also improved (4.6 to 2.8, p<0.001) . By study endpoint, 67.7% (21/31) of analyzed patients met MADRS response (≥50% reduction), and 74.2% were rated as responders on CGI-I. (Six patients dropped out in week 1, so 31 patients were evaluable.) These response rates are high for a TRD sample, suggesting a robust antidepressant effect.
- Side Effects/Dropouts: 10 of 37 patients (27%) discontinued pramipexole due to adverse events . Notably, 6 dropouts occurred in the first week of titration, likely from acute side effects such as gastrointestinal upset. Among those who tolerated initial titration, most completed 16 weeks (19 patients finished). Common side effects included nausea, headache, and somnolence (dose-dependent), manageable with slow titration.
- Mania/Hypomania Risk: The publication did not report any cases of mania or hypomania during the 16-week trial. Patients were on mood stabilizers and closely monitored; no mood switching was observed in the short term (consistent with other controlled trials where switch rates on pramipexole were low).
Cassano et al. (2004) – Extended 48-Week Follow-Up Study
- Study Type/Year: Long-term open follow-up (2004) of adjunctive pramipexole in treatment-resistant depression. (This appears to be an extension of the Lattanzi 16-week trial.)
- Population: 23 patients (from an initial acute sample of 31) with refractory major depressive episodes who received pramipexole augmentation. Of these, 12 had unipolar depression and 11 had bipolar depression. Mean age was ~53; all had multiple prior depressive episodes.
- Pramipexole Dose: Continued adjunctive pramipexole for up to 48 weeks total. Doses ranged 0.375–1.5 mg/day; the mean dose during continuation was ~0.99 mg/day. Patients were seen at weeks 16, 32, and 48.
- Outcomes: Sustained remission (defined as a LIFE depression rating ≤2 for ≥8 weeks) was achieved by 60.9% (14/23) of patients during follow-up. Median time to remission was 10 weeks, with most remitters achieving it by week 22. However, relapse was common: 5 of 14 remitters (36%) had a depressive recurrence by weeks 24–30. Still, by week 48 a substantial subset maintained wellness. The authors concluded long-term pramipexole augmentation was “relatively safe and presumably effective” for chronic, resistant depression.
- Side Effects/Dropouts: No instances of sudden sleep attacks (a concern with dopamine agonists) were seen. The most significant adverse events were affective switches (see below). Otherwise, side effect frequency decreased over time, and no new safety issues emerged by 48 weeks. Adherence was good among those who reached remission (some relapsers likely discontinued).
- Mania/Hypomania Risk: Three patients had mood elevation episodes: two developed hypomania and one experienced a psychotic mania during continuation (at weeks 22, 24, and 30, respectively). All occurred after several months on pramipexole. The manic symptoms resolved upon pramipexole discontinuation. This 13% switch rate (3/23) over long-term exposure highlights that while rare, mania can occur, underscoring the need for monitoring in extended use.
Hori et al. (2012) – Open-Label Adjunctive Trial in Japan
- Study Type/Year: 12-week open-label trial (2012) testing pramipexole as add-on therapy in SSRI-resistant depression.
- Population: 17 patients with DSM-IV major depressive episodes who failed to respond to an SSRI. This included 12 with unipolar major depressive disorder (MDD) and 5 with bipolar II disorder. All continued their existing antidepressant (or mood stabilizer) and received adjunct pramipexole.
- Pramipexole Dose: Titrated gradually over 12 weeks to a mean maximum of 1.6 mg/day (SD 0.9) . Dosing was individualized based on tolerability.
- Outcomes: Depression severity (HDRS-21) improved significantly. Mean HDRS dropped from 19.4 at baseline to 7.2 at week 12 (p < 0.000001).
- Response (≥50% HDRS reduction) occurred in 71% of patients, and remission (HDRS <8) in 59%. Notably, when analyzing only the unipolar subset, results were similar, suggesting bipolar II patients benefited comparably. This open trial thus supported a robust antidepressant effect for pramipexole augmentation in both bipolar II and unipolar depression.
- Side Effects/Dropouts: No serious adverse events were observed . Most side effects were mild; transient nausea and sleepiness were reported but did not lead to dropout. All 17 patients completed the trial, indicating excellent tolerability in this small sample.
- Mania/Hypomania Risk: No instances of mania or hypomania occurred in this 12-week study. The authors specifically noted that pramipexole was “effective and well tolerated” with no patients developing mood-switching or impulsivity problems .
Fawcett et al. (2016) – High-Dose Pramipexole Case Series (Long-Term)
- Study Type/Year: Case series (2016) reporting clinical experience with high-dose pramipexole in refractory depression.
- Population: 42 patients (age 25–81) with treatment-resistant depressive episodes: 24 unipolar MDD and 18 bipolar (I or II) depression. All had failed at least 4 prior antidepressant trials, reflecting highly resistant illness. Pramipexole was used as an augmentation to ongoing treatment.
- Pramipexole Dose: Higher dosing was pursued to maximize effect. The mean effective dose was 2.46 mg/day , with many patients in the 2.0–3.5 mg/day range. Titration was slow to improve tolerability, and some patients went above 3 mg if needed.
- Outcomes: 76% of patients (32/42) achieved a meaningful clinical improvement. Specifically, 20 patients (48%) reached full remission and 12 (29%) had a partial response by clinician judgment. Only 2 patients (5%) had no therapeutic response. These outcomes are remarkable given the level of treatment resistance, though the authors caution that “response” was based on clinical impression rather than strict rating scales. Nonetheless, it suggests a high proportion benefited when pramipexole was pushed to higher doses.
- Side Effects/Dropouts: Nausea was the dose-limiting side effect in many cases. 8 patients (19%) could not tolerate pramipexole due to severe nausea/vomiting that typically emerged within the first 3–10 days of treatment. In those cases, the drug was discontinued. Apart from gastrointestinal effects, other side effects (sedation, dizziness) were generally mild and manageable. No impulse-control problems (e.g. gambling, hypersexuality) were seen in this cohort (such behaviors are reported in Parkinson’s disease patients on pramipexole, but not observed here).
- Mania/Hypomania Risk: The case series did not report any incidences of mania or hypomania. All bipolar patients were on mood stabilizers, which likely mitigated switch risk. Overall, long-term pramipexole was deemed feasible in this refractory sample, with the main challenge being early nausea rather than mood destabilization.
Meta-Analyses and Reviews
Several reviews have synthesized the above evidence, given the generally small sample sizes of individual studies:
Aiken (2007) – Systematic Review: An early review of pramipexole in psychiatry compiled data from 8 studies of mood disorder patients. Pooled discontinuation due to any cause was only ~9%, highlighting overall tolerability. Notably, Aiken found that in controlled trials of bipolar depression, pramipexole’s switch rate into mania/hypomania was comparable to placebo. Combining all bipolar patients on pramipexole (N=85 across trials) yielded an estimated short-term switch rate of ~5% for hypomania and 1% for mania – rates no higher (and possibly lower) than those seen with standard antidepressants. This review suggested pramipexole has a favorable risk-benefit profile as an antidepressant, though it stressed the need for larger samples to confirm long-term safety .
Tundo et al. (2019) – Systematic Review and Meta-Analysis: This meta-analysis (5 RCTs, 3 open trials, 5 observational studies; n=504 total) found an overall short-term response rate of ~52% and remission rate of 36% with pramipexole. Pooled analysis confirmed pramipexole was superior to placebo in RCTs (RR for response ~1.77, 95% CI 1.11–2.82). Notably, pramipexole’s efficacy was similar to standard antidepressants in the limited head-to-head data (no significant difference vs. SSRI, RR 0.93). In terms of acceptability, pramipexole-treated patients were not more likely to drop out than controls, and tolerability was generally good. The most frequent side effect was nausea, in line with individual trials. Importantly, no increase in impulsive behaviors (pathologic gambling, hypersexuality, etc.) was seen in depressed patients on pramipexole – these dopaminergic side effects appear specific to Parkinson’s disease populations. The authors concluded there is supportive evidence that pramipexole is an effective adjunct in unipolar and bipolar depression, though large definitive trials are still lacking.
Tundo et al. (2022/2023) – “Real-World” Meta (Augmentation in TR Depression): A more recent analysis stratified outcomes by diagnosis. The treatment response rate to pramipexole augmentation was ~66% in bipolar depression versus ~57% in unipolar depression, a difference that was not statistically significant. Remission rates were around 39% in bipolar vs 61% in unipolar (p=0.20), indicating numerically lower remission in bipolar samples but with overlapping confidence intervals. Pramipexole’s dropout rate due to adverse events pooled to about 11% , with no significant difference between bipolar and unipolar groups. This suggests that efficacy and tolerability are broadly similar in bipolar I/II vs. unipolar depression when pramipexole is used as an adjunct.
Efficacy in Bipolar I vs. Bipolar II Depression
The evidence base for pramipexole spans both bipolar I and bipolar II depression, but direct comparisons are limited:
Bipolar II Depression: Pramipexole may be especially promising in bipolar II, as this diagnosis is often characterized by severe depressive episodes with prominent anhedonia. The dedicated RCT in bipolar II patients (Zarate 2004) showed a significant antidepressant effect. Additionally, an open-label series in bipolar II (Perugi 2001) and inclusion of bipolar II cases in other trials (Hori 2012, Fawcett 2016) demonstrated substantial response rates. Notably, bipolar II patients have, by definition, a lower risk of full manic switches, which might make clinicians slightly more comfortable using adjunctive antidepressant strategies. Pramipexole’s observed switch rate in bipolar II appears low (Zarate et al. saw only 1 hypomania on drug vs 2 on placebo). Thus, the evidence – though limited – indicates pramipexole is efficacious in bipolar II depression and does not commonly precipitate hypomania when combined with a mood stabilizer.
Bipolar I Depression: Patients with bipolar I were included in Goldberg et al. 2004 and in many open-label studies (Lattanzi 2002, Cassano 2004, Fawcett 2016, PAX-BD 2023). These collectively suggest pramipexole is also effective in bipolar I depression. For instance, Goldberg’s RCT (mixed BP I/II) had a large effect size (response 67% vs 20%). None of these studies signaled a dramatically higher switch risk in bipolar I, but sample sizes were small. In the long-term Cassano follow-up, the one case of psychotic mania could imply that bipolar I patients (who can develop full mania) require vigilant monitoring. Still, the overall impression is that bipolar I depressed patients derive antidepressant benefit from pramipexole similar to bipolar II patients. The meta-analytic data did not find a significant outcome difference between bipolar vs. unipolar subsets, indirectly supporting that bipolar I and II respond to pramipexole on par with unipolars.
Comparative Efficacy: There is no head-to-head trial of pramipexole in bipolar I vs. II, so any efficacy difference is speculative. Clinically, both bipolar I and II depression present with dopamine-linked symptoms (low motivation, anhedonia) that pramipexole may target. Some experts have suggested pramipexole might be particularly useful in bipolar II or in bipolar I patients who do not have rapid cycling or mixed features. Overall, the drug appears to have antidepressant efficacy across bipolar subtypes. Where evidence is stronger (bipolar II has one dedicated RCT) it is positive, and there is no sign that bipolar I patients fare worse – they were helped in combined studies, albeit with usual precautions. The key distinction lies not so much between bipolar I vs II in responsiveness, but in the safety profile: bipolar I patients inherently carry a higher mania risk, so pramipexole must be used with a bit more caution in that group.
Clinical Use and Consensus Guidance
Position in Therapy: Pramipexole is not a first-line treatment for bipolar depression but is recognized as a useful off-label option, especially in treatment-resistant cases. Psychiatric consensus and guidelines (while not officially listing pramipexole due to limited FDA approval) acknowledge its potential when standard treatments (e.g. mood stabilizers, atypical antipsychotics, antidepressants with caution) have failed. For example, an expert review in Psychiatric Timescalls pramipexole “an underutilized option” for bipolar depression, particularly to address prominent low energy and anhedonia. It is often considered at the stage of third-line augmentation – after trying approved options like quetiapine, lurasidone, lithium, lamotrigine, etc.
Patient Selection: The ideal candidate is a bipolar I or II patient with a depressive episode without mixed features or psychosis, who has not responded to first-line therapies. Because pramipexole can be activating, it is best suited for bipolar depression characterized by psychomotor slowing, apathy, and anhedonia (rather than anxiety or insomnia). Clinicians also ensure a mood stabilizer is in place (lithium, valproate, or an atypical antipsychotic) before adding pramipexole, to mitigate switch risk. Avoiding use in patients with a recent history of rapid cycling or manic instability is prudent until more data confirm long-term safety.
Dosing and Titration: Slow titration is critical to tolerability. A commonly recommended schedule is to start at 0.125–0.25 mg at night, then increase by 0.25 mg every 5–7 days as tolerated. Evening dosing is often preferred (pramipexole can cause drowsiness), though if it proves activating or disrupts sleep, morning dosing can be tried. Clinicians are advised to monitor progress using rating scales and reassess once the dose reaches ~0.75 mg/day, as some patients respond at low doses. Typical effective doses in bipolar depression are around 1.5 mg daily (given at bedtime), though cases may require pushing into the 2–3 mg range for full effect. High-dose strategies (2–4 mg) have been used in refractory depression with good efficacy, but side effects increase with dose. If side effects (like nausea) emerge, dose escalation should pause or retreat slightly until symptoms resolve. Using anti-nausea measures (e.g. ondansetron or ginger supplement) can help patients stay on the drug during titration .
Monitoring and Safety: Key aspects to monitor are mood switches, impulsive behaviors, and common side effects. Patients and families should be educated to watch for any signs of hypomania or mania (decreased need for sleep, racing thoughts, impulsivity). Fortunately, short-term controlled data suggest the risk of mania is low when pramipexole is used with a mood stabilizer. One meta-analysis estimated only ~1% risk of mania and ~5% hypomania in bipolar patients on pramipexole (over ~3 months), which is actually lower than the switch rate seen with standard antidepressants. Nonetheless, caution is warranted, especially in bipolar I: regular follow-ups (e.g. weekly or biweekly early in treatment) are recommended to detect mood elevation early.
Other safety points include the potential for sedation or sleep attacks – patients should be advised not to drive if they feel very sleepy (though frank “sleep attacks” are more an issue at higher doses in Parkinson’s disease). Impulse control problems (pathological gambling, hypersexuality, compulsive shopping) are rare in psychiatric use; these were not reported in mood disorder trials, but clinicians remain attentive to any new-onset compulsive behaviors. In practice, such side effects are seldom seen at the doses used for depression.
Side Effect Management: The most common pramipexole side effects in bipolar depression trials were nausea, fatigue, and somnolence. Nausea is typically transient, peaking in the first 1–2 weeks. Taking the dose with food, using proton-pump inhibitors or low-dose ondansetron as needed, and slow upward titration can alleviate nausea. If insomnia or vivid dreams occur (due to dopaminergic activation), shifting the dose to morning may help. Orthostatic dizziness can happen, so patients should rise slowly from sitting/lying positions. Unlike many psychiatric medications, pramipexole does not cause weight gain, sexual dysfunction, or cognitive blunting, which makes it attractive in patients who cannot tolerate those effects from other meds. In fact, its side effect profile is considered “clean” aside from dopaminergic effects like nausea. Rarely, edema can occur in older patients , and there is a theoretical link to cardiac valvulopathy or heart failure in Parkinson’s populations, though evidence is mixed and caution is advised in patients with severe cardiac conditions .
Duration of Therapy: Because data on long-term use in bipolar depression are limited, clinicians individualize duration. Some experts continue pramipexole for 6–12 months after remission before considering a taper. In practice, there are reports of patients maintained for years. Dr. Chris Aiken, for example, notes seeing no tolerance or mood cycling in patients kept on pramipexole for 10+ years in his clinic. Nonetheless, periodic re-evaluation is wise. If a decision is made to discontinue, it should be tapered over 1–2 months to avoid withdrawal or dopamine rebound symptoms .
Consensus and Conclusion: In summary, pramipexole has emerged as a valuable off-label tool for bipolar depression, backed by multiple positive small trials and case series. It consistently shows antidepressant efficacy in both bipolar I and II depression, with response rates around 50–70% in treatment-resistant samples. The risk of inducing mania or hypomania is relatively low when used as an adjunct to mood stabilizers, especially compared to standard antidepressants, but it is not zero – careful patient selection and monitoring are required. Side effects like nausea can limit use for some, but are often manageable with gradual dosing. There is a growing clinical consensus that pramipexole is an appropriate option for bipolar depression with prominent anhedonia or treatment resistance, typically as a third-line adjunct. Guidelines (e.g., CANMAT/ISBD 2018) mention dopamine agonists as experimental options for difficult bipolar depression, and recent reviews encourage its use in the “half-tested world” of off-label therapies when conventional measures fail. In practice, pramipexole should be used by experienced clinicians as part of a comprehensive bipolar management plan. Its evidence base, while still developing, is bolstered by meta-analytic support and over two decades of accumulated clinical experience. Overall, pramipexole offers a beacon of hope for patients with bipolar I or II depression who have not achieved remission with standard treatments, providing meaningful improvements in mood and motivation in a subset of these difficult-to-treat patients.
Sources:
Aiken 2020 (Psychiatric Times)
Disclaimer
This document is for informational and educational purposes only. It does not constitute medical advice, diagnosis, or treatment. The use of pramipexole and other medications discussed here is off-label and not FDA-approved for bipolar depression. Clinical decisions should be made in consultation with a licensed healthcare provider. The authors and OffLabel.ai assume no responsibility for outcomes resulting from application of this information without professional oversight. Use at your own risk.
No Comments.