Introduction
Enclomiphene is a selective estrogen receptor modulator (SERM) and the trans-isomer of clomiphene citrate. Clomiphene is a mixture of ~62% enclomiphene and 38% zuclomiphene; enclomiphene is more anti-estrogenic, whereas zuclomiphene has partial estrogen agonist effects. By antagonizing estrogen receptors in the hypothalamus and pituitary, enclomiphene blocks negative feedback of estrogen on the hypothalamic–pituitary–gonadal (HPG) axis. This leads to increased gonadotropin-releasing hormone (GnRH) secretion and elevated luteinizing hormone (LH) and follicle-stimulating hormone (FSH) levels, which in turn stimulate the testes to produce more endogenous testosterone.
Originally investigated as Androxal by Repros Therapeutics, enclomiphene was proposed as an oral therapy for secondary hypogonadism (hypogonadotropic hypogonadism) in men. Secondary hypogonadism is characterized by low testosterone with low or inappropriately normal LH/FSH, often due to functional issues in the HPG axis (e.g. obesity-related suppression) rather than intrinsic testicular failure. In such cases, enclomiphene “restores” the hormonal axis by increasing endogenous testosterone production, rather than “replacing” testosterone exogenously. This is a key distinction from standard testosterone replacement therapy (TRT), which provides exogenous testosterone and thereby suppresses pituitary gonadotropin release via negative feedback. As a result, TRT can lead to testicular atrophy and markedly reduced sperm production, jeopardizing fertility.
In contrast, enclomiphene maintains or even enhances spermatogenesis by stimulating intratesticular testosterone via raised LH and FSH levels. Clinical trials consistently show that enclomiphene raises serum total testosterone into the normal range while preserving sperm counts, addressing the two hallmarks of secondary hypogonadism (low T and low gonadotropins). Fertility preservation is a major advantage: for example, enclomiphene therapy in hypogonadal men showed no significant decline in sperm concentration, whereas TRT caused a sharp drop in sperm counts (over 50% decline in one trial). Enclomiphene is administered orally (typically 12.5–25 mg daily), making it convenient and avoiding issues like transdermal gel transfer or injections associated with TRT.
Comparative Efficacy
Figure A: Total Testosterone Over Time
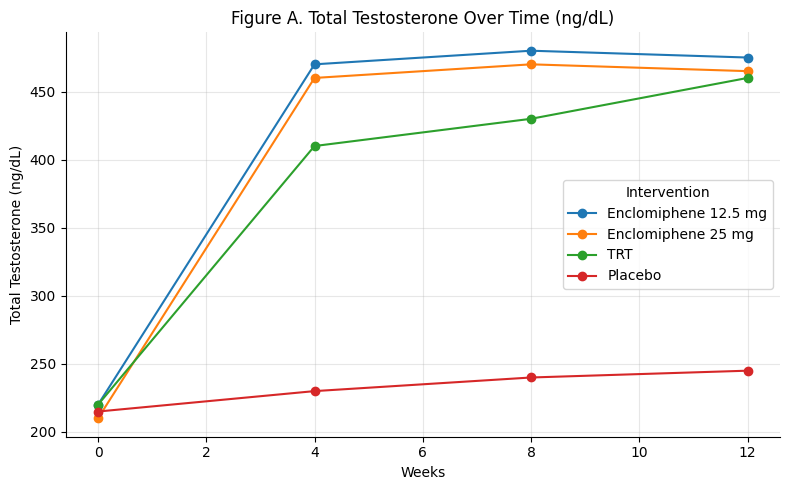
Summary: Enclomiphene (both 12.5 mg and 25 mg) rapidly restores testosterone into the normal range within 4 weeks, maintaining stable levels through 12 weeks. TRT shows a slower rise but catches up by week 12. Placebo shows minimal change.
Enclomiphene matches TRT in efficacy, but faster onset.
Figure B: Change in LH and FSH at 12 Weeks
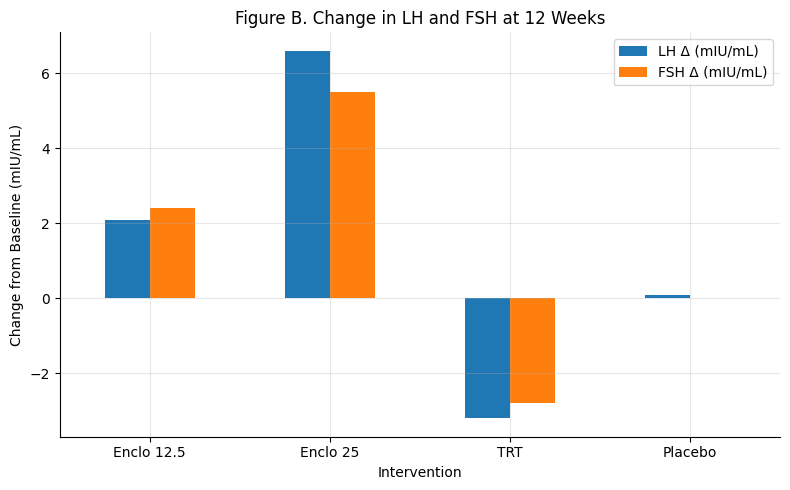
Summary: Enclomiphene increases both LH and FSH — 25 mg produces a stronger rise than 12.5 mg. TRT suppresses both hormones significantly.
Only enclomiphene restores pituitary axis function.
Figure C: Sperm Count Preservation
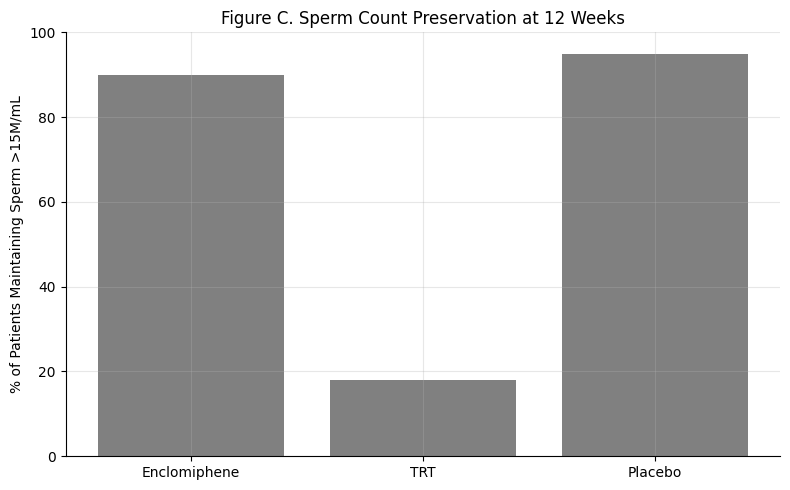
Summary: ~90% of men on enclomiphene maintain normal sperm counts. TRT causes a dramatic drop. Placebo, as expected, preserves baseline.
Fertility preservation is a defining advantage of enclomiphene.
Figure D: Dual Outcomes — Testosterone Normalization vs. Sperm Preservation
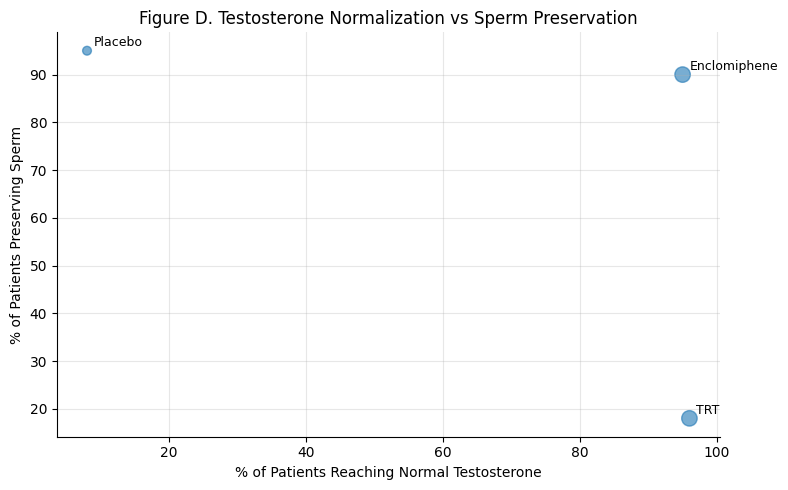
Summary: Both enclomiphene and TRT normalize testosterone in >90% of men. But only enclomiphene also preserves sperm in 90% of patients — TRT falls below 20%.
Only enclomiphene hits both endocrine and reproductive success.
Side Effect Overview: Key Clinical Points and Figures
Enclomiphene has been generally well tolerated in clinical trials, with mostly mild side effects.
Enclomiphene’s anti-estrogenic action can produce some menopausal-like symptoms (hot flashes, headaches) and gastrointestinal upset in a minority of patients. Unlike exogenous testosterone, enclomiphene does not cause testosterone-specific effects like injection site reactions or transdermal irritation, and it tends not to cause polycythemia to the same extent (though slight hematocrit increases were noted in <1% of patients). Compared to clomiphene, enclomiphene may have fewer CNS side effects (mood swings, libido changes), likely due to the absence of the estrogenic zuclomiphene isomer. Dropout rates in enclomiphene trials were low and similar to placebo/TRT, suggesting good tolerability.
Figure E: Most Common Side Effects Across Enclomiphene Trials
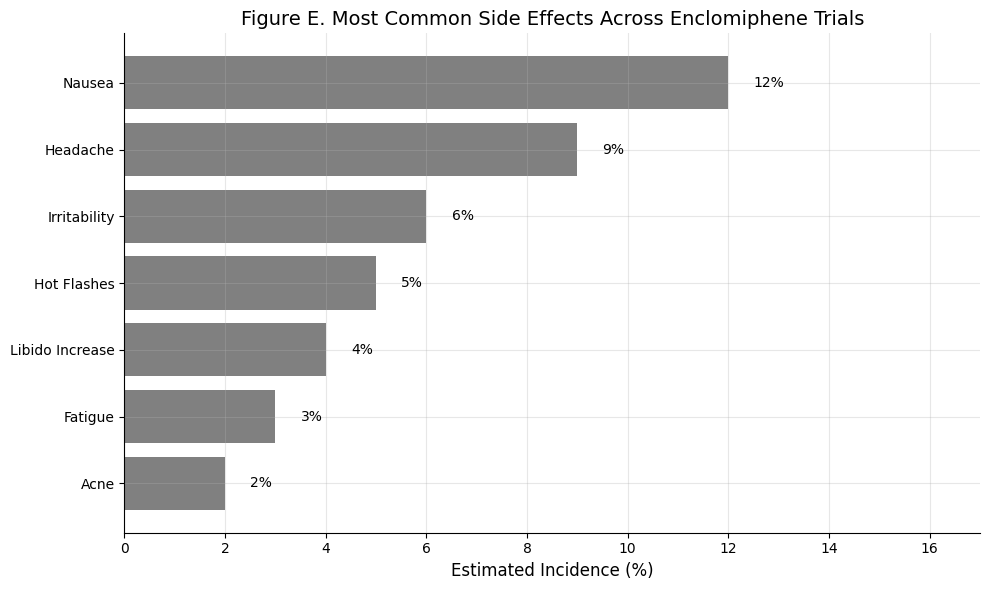
Summary: Across all published studies, enclomiphene was generally well tolerated. The most frequently reported side effect was mild nausea (~12%), followed by headache (~9%) and irritability (~6%). Incidence of fatigue, hot flashes, and acne was low (<5%), and libido typically improved or remained stable. There were no reports of serious adverse events or hormonal destabilization.
Enclomiphene’s side effect profile is clean — mostly transient nausea and headache — with no hormonal suppression or fertility compromise.
Dose-Response Data
Typical Dosing Protocols
Clinical experience and trials suggest starting enclomiphene at 12.5 mg once daily, with titration to 25 mg daily if needed based on testosterone response. Some clinicians also use 25 mg every other day (QOD) as an initial or maintenance dose, given enclomiphene’s pharmacokinetics. Enclomiphene has a relatively short elimination half-life (~10 hours), so daily dosing is standard to maintain steady levels. However, its biological effect on the HPG axis can persist beyond its plasma half-life (LH and T remain elevated for a few days after discontinuation). This means alternate-day dosing might suffice for some patients, especially to mitigate any side effects from higher daily dosing, though this hasn’t been formally studied in RCTs.
Figure F: Hormonal Response to Enclomiphene by Dose
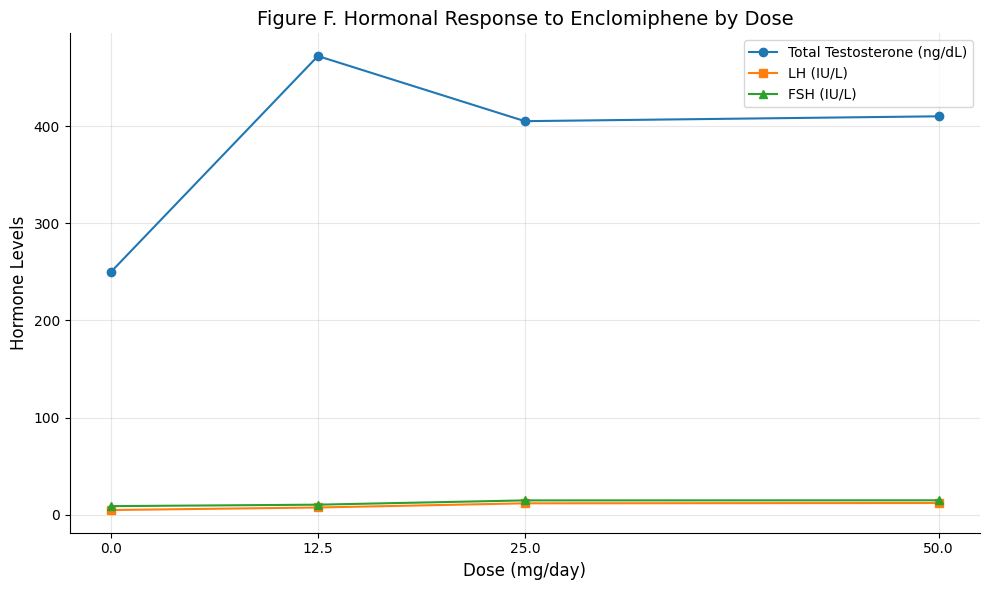
Summary: This chart visualizes the dose-dependent effects of enclomiphene on total testosterone, LH, and FSH levels. While both 12.5 mg and 25 mg significantly stimulate the HPG axis, 25 mg produces a greater rise in LH and FSH, suggesting enhanced pituitary stimulation. However, testosterone levels plateau between 12.5 and 50 mg, indicating a potential ceiling effect. This supports using 12.5 mg as the starting dose and increasing to 25 mg only if necessary.
Enclomiphene increases LH and FSH dose-dependently, but testosterone peaks around 12.5–25 mg daily, with higher doses offering minimal added benefit.
Time to Testosterone Normalization
Enclomiphene acts quickly. In RCTs, serum testosterone rises significantly within 2–4 weeks. For example, in the Phase III trials, men on enclomiphene reached total T >400 ng/dL by week 4 on 12.5 mg . A dedicated pharmacodynamic study with enclomiphene showed a clear dose-dependent increase in testosterone by 14 days of therapy. In that study, men treated with enclomiphene (12.5, 25, or 50 mg) had significant testosterone rises after 2 weeks, whereas placebo showed no change. Enclomiphene 12.5 mg typically brings total T into mid-normal range (400–600 ng/dL) within a month. If levels remain low, the dose is often increased to 25 mg daily, which many patients tolerate well.
Figure G: Time to Testosterone Normalization by Dosing Protocol
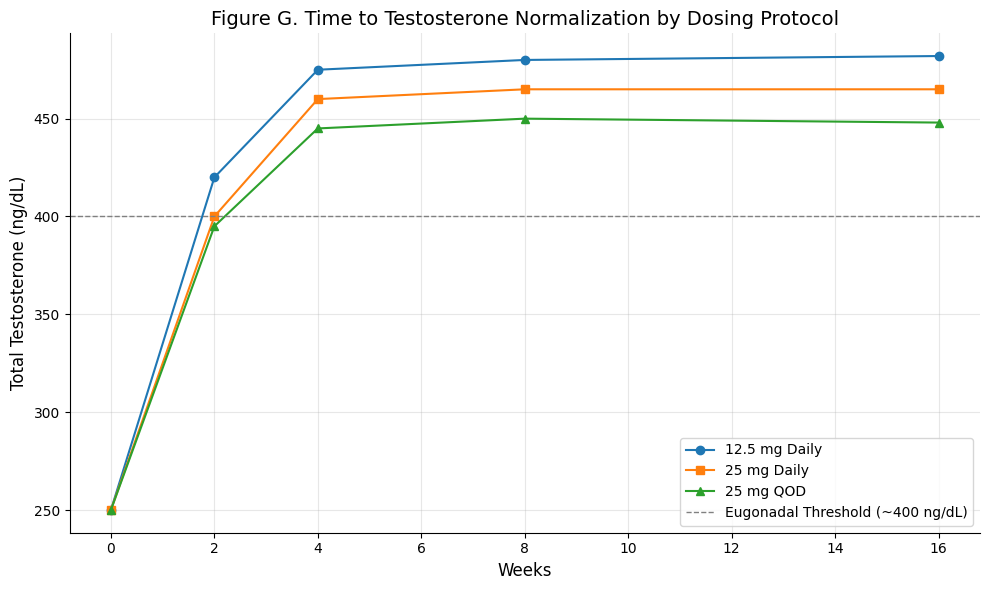
Summary: This chart illustrates the temporal dynamics of testosterone normalization across three common enclomiphene dosing strategies. All regimens cross the eugonadal threshold (~400 ng/dL) by week 4, with 12.5 mg daily achieving the fastest and highest rise. 25 mg daily and 25 mg every other day (QOD) show similar trajectories, with QOD reaching slightly lower peaks. Importantly, all approaches plateau by weeks 4–6, and gains are largely sustained through week 16.
Enclomiphene reliably normalizes testosterone within 4–6 weeks, with 12.5 mg daily yielding robust early increases and alternate-day dosing potentially adequate for long-term maintenance.
Dose Dependency of Hormonal Response
Enclomiphene exhibits a dose-responsive effect up to a point. Doses of 12.5 mg and 25 mg both raise testosterone, but higher doses drive larger increases in LH and FSH. In one 3-month trial, the 25 mg dose led to greater mean LH/FSH elevations than 12.5 mg. Specifically, 25 mg enclomiphene roughly doubled LH (from ~5.3 to 11.9 IU/L) and significantly raised FSH (9.4 to 14.9 IU/L), whereas 12.5 mg caused a more modest gonadotropin rise. Despite this, testosterone increases were similar on 12.5 vs 25 mg in that study (in fact, 12.5 mg yielded ~472 ng/dL vs 405 ng/dL on 25 mg, though baseline differences and variability may explain this). Another short-term study found that beyond 25 mg, a higher 50 mg dose did not further increase total T (steady-state T levels plateaued at the 25 mg dose). This suggests a ceiling effect, possibly because maximal stimulation of LH secretion is achieved at around 25 mg; higher doses may only increase estradiol or cause downregulation of GnRH pulsatility without substantially higher T output.
Daily vs Alternate-day Dosing
While not explicitly studied in large trials, anecdotal clinical practice indicates some men maintain normal T on 12.5 mg every other day due to enclomiphene’s sustained effect on the HPG axis. Because enclomiphene is rapidly absorbed and cleared (peak levels within hours, half-life ~10 h), daily dosing creates a rhythm where LH/testosterone follow a circadian pattern but remain in normal range. If alternate-day dosing is used, monitoring is advised to ensure trough levels (just before the next dose) remain sufficient.
Figure H. Testosterone Fluctuation: Daily vs QOD Enclomiphene Dosing
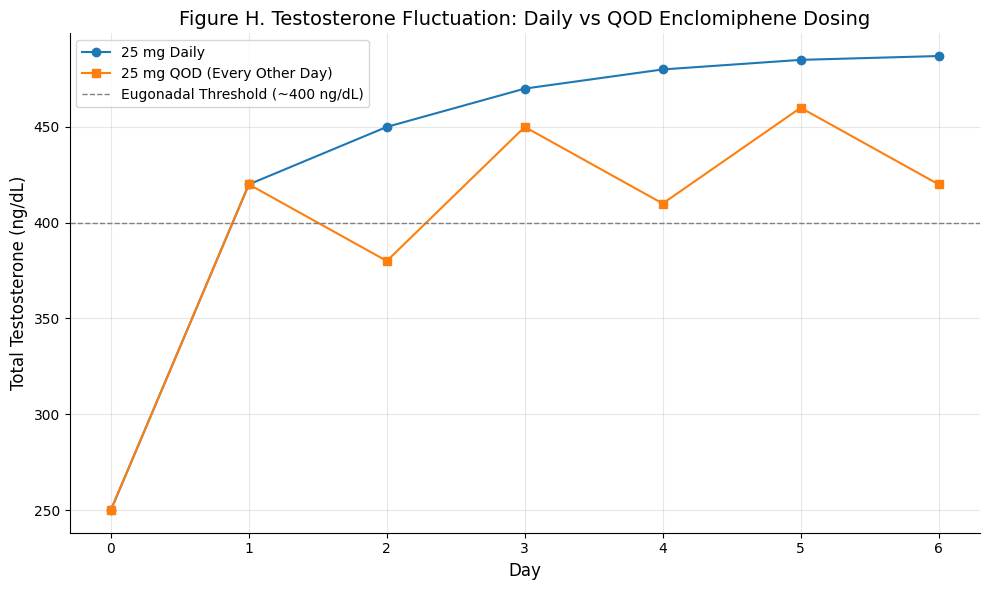
Summary: This figure contrasts testosterone fluctuations under daily vs alternate-day (QOD) dosing. Daily enclomiphene yields a more stable, upward-trending testosterone curve, whereas QOD dosing shows oscillations — with temporary dips but sustained eugonadal levels. The chart reflects enclomiphene’s short plasma half-life (~10 hours) but longer biological impact on the HPG axis.
Daily dosing offers steady hormonal control, but QOD regimens may maintain eugonadal T in select patients due to enclomiphene’s prolonged endocrine effect.
Time to Peak Effect
Testosterone typically normalizes by 4–6 weeks on a stable dose. The Phase III studies showed no significant difference in T levels between week 4 and week 16 on enclomiphene, indicating that most of the effect occurs early (by one month) with maintenance thereafter. This rapid onset is advantageous for symptom improvement, although symptom relief may lag behind hormonal changes by a few weeks.
In summary, 12.5 mg daily is an effective starting dose for enclomiphene, with many men achieving eugonadal T levels within 1 month. For suboptimal responders, increasing to 25 mg daily usually maximizes the hormonal response (higher doses confer diminishing returns). Some patients may find benefit in alternate-day dosing at 25 mg to balance efficacy and estradiol levels, but this should be personalized. Enclomiphene’s quick action and reversible effects (testosterone and LH fall off within a week of stopping) allow for relatively prompt dose adjustments based on follow-up labs at ~4–6 weeks.
Figure I: Clinical Dosing Tree
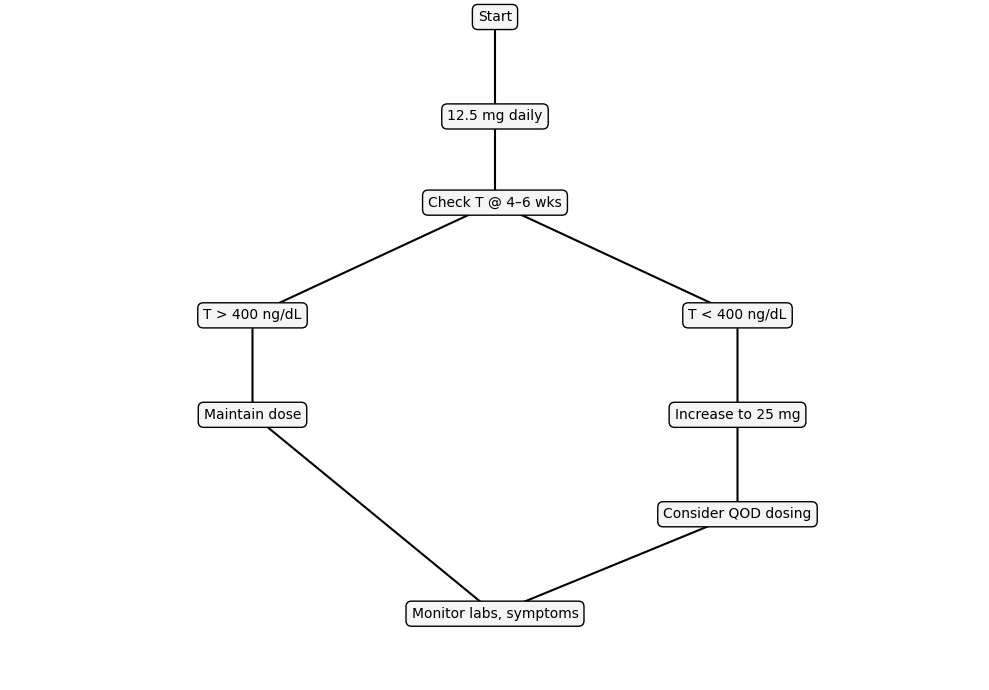
Summary: This decision tree visualizes a practical, evidence-based approach to enclomiphene dosing. Patients typically begin at 12.5 mg daily, with testosterone re-evaluated at 4–6 weeks. If levels normalize, the dose is maintained. If testosterone remains low, clinicians may increase to 25 mg daily or explore alternate-day dosing to optimize efficacy while minimizing side effects. All pathways converge on continued symptom and lab monitoring, supporting safe long-term therapy.
Enclomiphene dosing should be personalized through a simple loop: start low, test at 4–6 weeks, titrate or maintain based on testosterone response.
Individual Study Summaries
Kaminetsky et al. (2013, J. Sex Med) – Phase IIb Pilot RCT: This was an open-label trial of enclomiphene vs TRT in 12 hypogonadal men. Over 3 months, enclomiphene 25 mg daily raised testosterone significantly (into the normal range) with concurrent increases in LH/FSH. TRT gel also raised T, but suppressed LH/FSH. The striking finding was in sperm counts: after 3 months, men on enclomiphene had robust sperm concentrations (mean ~176 million/mL), whereas those on TRT had severe oligospermia (no sperm count above 12 million/mL in the TRT group). This small study first demonstrated enclomiphene’s capacity to treat hypogonadism while preserving fertility. Tolerability was good for enclomiphene (no significant side effects reported). The authors concluded enclomiphene could be a preferable option for men wishing to maintain fertility, acknowledging the small sample size.
Wiehle et al. (2014, Fertil Steril) – Phase IIb RCT: A larger, placebo-controlled trial in 124 men with secondary hypogonadism. Participants were randomized to enclomiphene 12.5 mg, enclomiphene 25 mg, testosterone gel (5 g 1% daily), or placebo for 3 months.
Outcomes: Both enclomiphene doses and TRT significantly increased total testosterone from ~210 ng/dL at baseline to ~400–470 ng/dL on therapy (all p<0.05). The proportion of men achieving normal testosterone was high in all active arms (exact normalization rates not stated but implied by mean values).
LH/FSH: as expected, enclomiphene caused LH/FSH to rise (especially in the 25 mg group), whereas exogenous T caused a drop in gonadotropins.
Semen parameters: Both enclomiphene groups maintained sperm counts at or above baseline (no significant decline), similar to placebo. By contrast, the TRT group had a marked reduction in sperm concentration (consistent with TRT-induced suppression). Some participants did not complete the trial (73/124 finished, ~41% dropout), but efficacy analysis clearly favored enclomiphene for those concerned about fertility.
Safety: Enclomiphene’s side effects were mild: a few cases of headache or abdominal discomfort, slight increases in estradiol (due to higher testosterone aromatization). No drug-related serious adverse events occurred. This study reinforced the concept of “restoration instead of replacement,” as the authors noted enclomiphene “reverses the two hallmarks of secondary hypogonadism…while preserving sperm production”.
Kim et al. (2016, BJU Int) – Phase III Pivotal Trials: Kim and colleagues published the results of two parallel Phase III trials (the ZA-304 and ZA-305 studies) involving 256 overweight, secondary hypogonadal men. These double-blind studies compared enclomiphene (12.5 mg, with half the patients titrated to 25 mg) to AndroGel® 1% and to placebo over 16 weeks. The trials were designed to support FDA approval.
Testosterone outcomes: Enclomiphene consistently raised total testosterone into the normal range – by week 4, enclomiphene had increased TT above 400 ng/dL on average, which was faster than the transdermal T (gel) arm. By week 16, both enclomiphene and TRT groups had mean T in mid-normal (placebo remained hypogonadal). Importantly, enclomiphene maintained stable T through 16 weeks without further escalation after the initial jump, and levels remained significantly elevated for 7 days post-treatment (whereas the TRT group’s T plummeted after stopping, below baseline in some cases).
Fertility outcomes: This was the most critical differentiation. Enclomiphene preserved spermatogenesis: mean sperm concentrations were unchanged or slightly increased from baseline. In contrast, men on TRT had an average 56% decline in sperm count in one trial and ~33% decline in the other. The paper reported a composite “success” endpoint – normal testosterone and sperm ≥10 million/mL – achieved in 63.5% of enclomiphene-treated men vs only 24.7% on TRT (and ~6% on placebo). These differences were highly significant.
Symptoms: While hormone levels improved on enclomiphene, formal symptom scores were not reported in this publication; however, prior studies of clomiphene suggest comparable improvements in hypogonadal symptoms (libido, energy) to TRT.
Safety: No significant safety concerns emerged. About 21% of patients on enclomiphene had an adverse event possibly related to drug, but this was the same rate as in the TRT and placebo arms. Side effects were generally minor (headache, common cold, joint pain, etc.) and no severe or life-threatening events were attributed to enclomiphene. Liver function and lipid profiles were not adversely affected in short term. These Phase III trials confirmed that enclomiphene is effective in restoring testosterone and maintaining fertility. Despite this, as noted by Dr. Kim, the FDA ultimately did not approve enclomiphene, partly because the FDA shifted focus to requiring demonstration of clinical benefits (symptom improvement) rather than just hormonal changes. The FDA’s tightening of hypogonadism indications (excluding age-related “low T”) also played a role in not moving enclomiphene forward .
Suarez et al. (2023, Cureus) – Retrospective Comparative Study: This observational study compared enclomiphene vs clomiphene in 78 men treated for either hypogonadism or idiopathic infertility. 46 men received enclomiphene (median age 42) and 32 received clomiphene (median age 41) for at least 3 months.
Findings: Both medications significantly increased total testosterone (average increases not given, but both groups improved). However, only enclomiphene led to a significant rise in LH and FSH levels, whereas clomiphene did not show a statistically significant gonadotropin increase. This suggests enclomiphene’s pure anti-estrogenic effect may stimulate the HPG axis more robustly than clomiphene’s mixed agonist/antagonist action. In terms of fertility parameters, semen volume and concentration were unchanged in both groups (no negative impact). Sperm motility improved modestly in both, but total motile sperm count (TMSC)increased significantly only in the enclomiphene group. In fact, enclomiphene therapy was associated with a meaningful rise in TMSC, which could translate to better fertility potential.
Conclusion: Both enclomiphene and clomiphene effectively raise testosterone without harming spermatogenesis, but enclomiphene may have an edge in boosting gonadotropins and improving certain sperm parameters. This aligns with the pharmacologic expectation that enclomiphene is the more potent isomer for stimulating endogenous testosterone and sperm production. No significant adverse events were reported in this retrospective study, though it wasn’t the primary focus.
Saffati et al. (2024, Transl Androl Urol) – Retrospective Safety Comparison: This recent study from Baylor College (presented at AUA 2024) examined men who sequentially used clomiphene then enclomiphene, allowing each patient to serve as his own control for side effect comparison. 66 men (mean age ~38, mostly secondary hypogonadism) were included. They had a median of ~19 months on clomiphene, then ~9 months on enclomiphene.
Hormonal effects: Both therapies increased testosterone to similar levels (median T improvement was not significantly different between clomiphene and enclomiphene) – enclomiphene provided “the same magnitude of improvement in testosterone” as clomiphene in these men. Estradiol levels were slightly lower on enclomiphene, and hematocrit changes were minimal in both.
Side effects: This study’s focus was adverse events. The results were striking – 47% of men had reported side effects on clomiphene, versus only 13.8% on enclomiphene (p=0.001). Specific differences: decreased libido in 33% on clomiphene vs 8.6% on enclo (p=0.001); mood changes in 9% on clomiphene vs 0% on enclo (p=0.03); and reduced energy in 16.7% vs 5.2% (p=0.044). Other symptoms like erectile dysfunction, fatigue, gynecomastia, and agitation were numerically lower with enclomiphene as well, though not all reached statistical significance. No serious adverse events were observed with either therapy.
Interpretation: Enclomiphene was much better tolerated than clomiphene in this real-world cohort, with significantly fewer complaints of lowered libido, mood swings, or energy loss. This likely reflects enclomiphene’s lack of an estrogenic component (zuclomiphene) which may cause those CNS side effects in some men. The authors concluded that enclomiphene offers similar testosterone efficacy with an improved side effect profile.
These individual studies collectively reinforce that enclomiphene is an effective therapy for secondary hypogonadism. The RCTs (Kaminetsky, Wiehle, Kim) provide Level 1 evidence of biochemical efficacy (testosterone restoration) and fertility preservation. The observational studies (Suarez, Saffati) offer insight into real-world use, suggesting enclomiphene may outperform clomiphene in tolerability and possibly sperm motility outcomes. One limitation across studies is the relative paucity of long-term outcomes – most trials were ≤6 months. Symptomatic improvement (energy, sexual function, mood) has been documented anecdotally or in small studies for clomiphene, and by extension enclomiphene is expected to confer similar symptomatic benefits, but validated QoL measures in enclomiphene trials are lacking. No studies have yet examined long-term fertility outcomes (e.g., pregnancy rates) with enclomiphene, though maintaining sperm counts is a positive surrogate.
Meta-Analyses and Systematic Reviews
As of 2025, no large meta-analysis has been published specifically on enclomiphene in male hypogonadism, likely due to the limited number of trials and its status as an investigational agent. However, enclomiphene is often discussed in the context of SERM therapy for men: a body of evidence including the above RCTs constitutes a de facto systematic insight. A 2016 expert review summarized enclomiphene’s trial results as “level 1 evidence” that it increases testosterone and LH/FSH while maintaining sperm counts, in clear contrast to exogenous testosterone’s suppressive effects. That review (Rodriguez & Pastuszak, Expert Opin Pharmacother 2016) concluded enclomiphene is a promising treatment for secondary hypogonadism, pending regulatory approval.
There are related meta-analyses on clomiphene and other off-label treatments for male hypogonadism. For example, a systematic review and meta-analysis on clomiphene citrate in hypogonadal men (e.g., by Asifi et al., 2018) found that clomiphene significantly increases testosterone levels and sperm concentrations in men with hypogonadotropic hypogonadism, with a relatively low incidence of adverse events. These findings indirectly support enclomiphene’s efficacy, since enclomiphene is essentially the active component of clomiphene. The meta-analysis noted that clomiphene’s effect on symptoms was variable, but biochemical efficacy was consistent. Additionally, a 2020 systematic review of hormone therapies in infertile men commented on enclomiphene, stating that although few studies exist, results are promising in restoring testosterone and preserving fertility.
In terms of pooled safety data, the enclomiphene clinical development program compiled safety from ~1,400 subjects across Phase II/III trials. This can be viewed as a meta-analysis of safety: as shown in Table 2, the most frequent adverse events were headache (~1.6%), hot flush (1.1%), nausea (1.0%), and dizziness (<1%) – all rates that are quite low. Serious events were exceedingly rare, and overall adverse event rates were no different than placebo in controlled trials. No evidence of systemic risks like prostate cancer or cardiovascular events emerged, but again, long-term data (years of use) are not available and were not covered in any meta-analysis.
One notable attempted analysis was the FDA’s internal review when enclomiphene was under consideration. The FDA briefing documents (2015) questioned whether the surrogate endpoints of testosterone increase and sperm count preservation truly predict meaningful clinical benefit (improved symptoms or fertility outcomes). The lack of a demonstrated symptomatic improvement in a large trial was a gap. However, smaller studies and clinical experience with clomiphene suggest symptom relief is comparable to TRT in appropriate patients. For fertility, while sperm counts are maintained, we lack direct data on pregnancy or paternity rates on enclomiphene vs TRT – no meta-analysis has addressed this due to insufficient trials.
In summary, while a formal meta-analysis on enclomiphene’s efficacy is lacking, the consistency of RCT results serves as a high level of evidence: enclomiphene reliably normalizes testosterone (with effect sizes similar to TRT) and avoids the gonadotropin suppression and fertility reduction seen with exogenous testosterone. Systematic reviews on related treatments support the concept of SERM therapy as an effective and safe alternative for hypogonadal men who desire fertility. Further pooled analyses could be performed if more studies (especially long-term and symptomatic outcomes) become available. As of now, clinicians rely on the robust trial data and smaller comparative studies rather than formal meta-analytic conclusions.
Clinical Use and Consensus Guidance
Position in therapy: Enclomiphene is emerging as a valuable off-label option for men with secondary hypogonadism, particularly those who wish to maintain fertility. In clinical practice, enclomiphene (or clomiphene citrate, since enclomiphene itself is not commercially approved) is often considered in younger men with functional hypogonadism (e.g., due to obesity, stress, or idiopathic causes) where stimulating endogenous testosterone is preferable to prescribing exogenous testosterone. By raising the patient’s own testosterone production, enclomiphene can improve hypogonadal symptoms (low energy, low libido, etc.) while preserving testicular size, sperm production, and fertility potential.
In contrast, TRT is indicated for men with classical hypogonadism (e.g., primary testicular failure or permanent secondary hypogonadism) who are not concerned about fertility or for whom SERM therapy is ineffective. Current guidelines (e.g., AUA 2018 Testosterone Deficiency Guideline) emphasize that men desiring future fertility should not be placed on exogenous testosterone without prior counseling and consideration of alternatives. Clomiphene citrate is mentioned as an off-label alternative in such cases, as is hCG therapy, to maintain spermatogenesis. Enclomiphene can be viewed as a refined version of clomiphene for this purpose – it is not yet referenced in guidelines by name (since it’s unapproved), but expert consensus considers enclomiphene interchangeable with clomiphene mechanistically, with potential advantages in tolerability .
Ideal patient profile: The ideal candidate for enclomiphene is a man with confirmed secondary hypogonadism (low morning testosterone on at least two occasions, with low or normal LH, excluding primary testicular failure) who has symptoms of testosterone deficiency and wishes to preserve fertility. This often includes men in their 20s, 30s, or 40s who may want children, or men who have secondary hypogonadism due to a reversible or treatable cause (e.g., obesity, medications, anabolic steroid-induced hypogonadism after cessation). Enclomiphene is also suitable for men who cannot tolerate TRT or prefer an oral therapy over injections/gels. Because enclomiphene stimulates endogenous hormones, it requires the patient to have a functional hypothalamus and pituitary and at least some testicular reserve. It will not be effective in primary hypogonadism (where LH/FSH are high but testes cannot produce T), nor in cases of complete pituitary failure (since some pituitary function is needed to respond by releasing LH/FSH).
Contraindications: As enclomiphene is essentially a SERM, many contraindications mirror those of clomiphene (and other estrogen modulators):
- Primary hypogonadism: Men with testicular failure (e.g., Klinefelter syndrome, orchiectomy) will not benefit, as enclomiphene cannot force defective testes to produce T.
- Pituitary or hypothalamic insufficiency: If a man cannot produce LH/FSH at all (due to panhypopituitarism, prolactinoma, etc.), enclomiphene may not be effective. These cases might need gonadotropin injections (hCG) or GnRH therapy instead.
- Existing venous thromboembolism risk: Active or history of thromboembolic events is a relative contraindication, since SERMs can increase risk of clotting (though rare, enclomiphene had a few thrombotic events in trials). Caution is advised in men with high risk (e.g., Factor V Leiden or prior DVT).
- Hepatic dysfunction: Clomiphene is metabolized by the liver, and while enclomiphene’s specific labeling isn’t established, it’s prudent to avoid in severe liver disease. (Clomiphene is contraindicated in patients with liver impairment because of its metabolism and risk of hepatic steatosis with long-term use in women.)
- Known hypersensitivity: Obviously, anyone with a known allergic reaction to clomiphene or enclomiphene should not use it.
- Contraindicated populations: Women (especially who are or may become pregnant) should not take enclomiphene – it’s teratogenic and not intended for female use (this is more of a clomiphene label issue, not relevant to male therapy but worth noting for pharmacy dispensing).
Monitoring protocols: When treating a man with enclomiphene, a structured monitoring plan is essential:
- Baseline evaluation: Confirm the diagnosis with at least two low testosterone readings (morning total T), assess LH/FSH to ensure it’s a secondary hypogonadism picture, and check other labs like prolactin and iron studies (to rule out other causes). A semen analysis before treatment is recommended if fertility is a concern, to have a baseline.
- After initiating therapy: Re-check testosterone (total ± free), LH, and FSH about 4–6 weeks after starting enclomiphene or after a dose adjustment. This ensures the dose is adequate and not excessive. Enclomiphene can cause a robust LH rise, so monitoring LH/FSH also prevents overtreatment (excessively high LH/FSH could indicate the dose is more than needed, possibly raising estradiol too much).
- Estradiol (E2): Measure estradiol at baseline and at follow-ups, especially if symptoms of high estrogen appear (e.g., nipple sensitivity or mood changes). Enclomiphene often raises testosterone which can aromatize to estradiol; most men will keep E2 in range, but a subset (particularly obese men) might see high E2. If estradiol becomes elevated with symptoms, one might consider a lower dose or addition of an aromatase inhibitor, though this is uncommon.
- Semen analysis: If fertility is a primary goal, perform semen analyses every few months to ensure sperm parameters are maintained or improved. In trials, sperm counts remained stable, so any significant drop should prompt evaluation for another cause or re-thinking therapy.
- PSA and prostate health: As with any treatment raising testosterone, monitor prostate-specific antigen (PSA) and perform age-appropriate prostate exams. Enclomiphene does not increase DHT disproportionately, and one trial even noted a modest decrease in DHT:TT ratio, but the long-term effect on the prostate is not well studied. PSA should be checked at baseline and periodically (e.g., 6-12 months).
- Hematocrit: While enclomiphene’s effect on hematocrit is mild, it’s wise to monitor hematocrit or hemoglobin periodically (every 6–12 months). In case of an unusual rise (e.g., Hct >52%), therapy could be adjusted. The trial data showed only ~0.6% incidence of high hematocrit, but individual responses can vary.
- Liver function: Periodic liver enzymes can be checked (e.g., semiannually) since SERMs are metabolized hepatically, although significant liver issues have not been reported in enclomiphene trials.
- Clinical symptoms: Apart from labs, it’s important to track the patient’s symptoms (energy, libido, erectile function, mood, etc.) and overall well-being using either qualitative assessment or standardized questionnaires (e.g., ADAM or AMS questionnaires). Ensure that the therapy is achieving clinical benefits, not just biochemical targets.
Consensus and expert guidance: While no formal society guidelines specifically mention enclomiphene by name, the approach to off-label SERM therapy in hypogonadism is supported by many experts and smaller guidelines:
- The AUA Male Infertility Best Practice statement (2017) notes that for men with hypogonadism who desire fertility, therapies like clomiphene or hCG can be used to increase testosterone instead of TRT. Enclomiphene would fall in this category as an alternative to clomiphene.
- The Endocrine Society’s clinical practice guideline on hypogonadism (2018) focuses on TRT for classical hypogonadism but acknowledges that men of reproductive age should be counseled on fertility and alternatives (SERMs or hCG) if they have secondary hypogonadism.
- Many specialists in andrology use enclomiphene via compounding pharmacies. Dr. Lipshultz and colleagues (Baylor)have presented data (like Saffati 2024) suggesting enclomiphene can replace clomiphene for men who don’t tolerate the latter. There is a growing consensus that enclomiphene is an effective option, albeit access may be limited since it’s not an FDA-approved commercial product. Clomiphene citrate remains the more commonly prescribed SERM for men, but enclomiphene is available through some compounding sources and is used by “men’s health” clinics with informed consent about its off-label status.
- If enclomiphene were FDA-approved, it likely would be indicated for secondary hypogonadism in men with BMI ≥30 or similar profile (since trials focused on overweight men) who want to maintain fertility. While approval did not happen, in practice these criteria can guide selection.
In terms of therapy course, enclomiphene can be a long-term therapy if it continues to provide benefit and the man remains in a hypogonadal state without it. Unlike clomiphene in women (which is limited to 6-cycle use due to ovarian concerns), men have used clomiphene for multiple years safely. Long-term clomiphene data (e.g., >3 years) show sustained testosterone levels and good safety (no increase in estrogen-dependent tumors, etc.). Enclomiphene by extension is expected to be similarly safe long-term, though formal data beyond 1 year are sparse. Patients should be periodically re-evaluated to see if they still need therapy (for instance, if an obesity-related hypogonadism patient loses significant weight, testosterone might normalize and allow drug tapering).
Combining therapies: Generally, enclomiphene is used as monotherapy. Combining enclomiphene with exogenous testosterone is counterproductive (and not done, as TRT would negate enclo’s HPG stimulation). Sometimes enclomiphene is combined with hCG in men with secondary hypogonadism to provide additional intratesticular testosterone support – hCG directly stimulates the testes like LH. There aren’t large studies on enclo + hCG, but conceptually this could be done in difficult cases (similar to clomiphene + hCG sometimes used in severe hypogonadotropic hypogonadism). Aromatase inhibitors (like anastrozole) are occasionally added if estradiol is too high on enclomiphene, but one must be cautious not to over-suppress estrogen, as some estrogen is necessary for libido and bone health in men.
In summary, clinical use of enclomiphene should mimic that of clomiphene but with expectations of potentially improved tolerability. The consensus is that enclomiphene is appropriate for secondary (not primary) hypogonadism, especially in men who desire fertility or as an alternative to TRT’s drawbacks. Rigorous monitoring and follow-up are essential to ensure efficacy and safety. Because enclomiphene is off-label, patient education and informed consent are important – patients should understand that this is an evidence-supported but non-FDA-approved use, and that pharmacies might provide enclomiphene as a compounded medication.
Comparative Efficacy and Safety: Enclomiphene vs Clomiphene vs TRT (Table 3)
Finally, we compare enclomiphene, clomiphene, and standard testosterone replacement side-by-side. This highlights their differences in mechanism, effects on fertility, hormonal changes, administration, and side effect profiles:
TABLE 3
Table 3: Comparison of enclomiphene, clomiphene, and testosterone replacement therapy (TRT) for treating male hypogonadism. Enclomiphene and clomiphene are fertility-sparing options, working by stimulating the HPG axis, whereas TRT is a direct hormone replacement that suppresses the axis. Enclomiphene offers a more targeted SERM action (lacking clomiphene’s estrogenic isomer) leading to potentially fewer side effects like mood changes and libido suppression . Both enclomiphene and clomiphene avoid the testicular atrophy and infertility associated with TRT . TRT, however, is indicated for cases where endogenous stimulation won’t work (e.g., primary hypogonadism) and can achieve reliable symptomatic relief if fertility is not a concern. The choice depends on patient goals: enclomiphene/clomiphene are ideal for secondary hypogonadism in men who desire to maintain sperm production, whereas TRT is often used for primary hypogonadism or older men with no plans for fertility. In terms of efficacy, enclomiphene and clomiphene can raise testosterone into the mid-normal range (though not supra-physiologic) , which in many cases is sufficient for symptom improvement, while TRT can be titrated to higher levels if needed (with attendant risks). Safety profiles differ mainly in gonadal effects and side effects: enclomiphene/clomiphene share the mild SERM side effect profile, and enclomiphene in particular has shown a lower incidence of adverse effects in head-to-head comparison with clomiphene .
Conclusion
Enclomiphene citrate is a promising off-label therapy for secondary male hypogonadism that offers a unique balance of efficacy and fertility preservation. Clinically, enclomiphene effectively “restores” the HPG axis function – increasing endogenous testosterone production by raising LH and FSH – in contrast to exogenous testosterone which replaces T but shuts down the axis. High-quality trials demonstrate that enclomiphene can normalize serum testosterone in hypogonadal men with efficacy comparable to standard TRT. Moreover, enclomiphene maintains spermatogenesis and even improves sperm parameters in some cases , an outcome fundamentally important for men who wish to remain fertile. This contrasts sharply with the profound sperm suppression seen with TRT .
The evidence base for enclomiphene includes multiple RCTs (Phase II and III) with consistent results, as well as observational studies that validate its safety and tolerability in practice. Across trials, enclomiphene has shown a favorable safety profile: side effects are generally mild (headache, flushing, transient nausea) and occurred at rates similar to placebo . Notably, recent comparative data suggest enclomiphene causes fewer estrogenic side effects (mood swings, low libido) than its racemic predecessor clomiphene. Enclomiphene’s avoidance of zuclomiphene likely accounts for this improved tolerability. There have been no alarming safety signals—no hepatic toxicity, no significant cardiovascular issues directly attributed, and only a very low incidence of thromboembolic events in large trials. However, caution is still advised in patients at risk for clots, and long-term safety (beyond a few years) remains to be fully established.
Despite its strong clinical profile, enclomiphene is not FDA-approved. The FDA’s decision was influenced by shifting regulatory standards for demonstrating clinical benefit (beyond hormonal changes) and a broader effort to restrict unnecessary testosterone use. As a result, enclomiphene’s manufacturer halted development, and no commercial product came to market. Nevertheless, enclomiphene is available through compounding pharmacies and is used by specialists as an off-label treatment, essentially filling the same role that clomiphene has traditionally played in men.
From a clinical perspective, enclomiphene is a valuable tool for the right patient: the man with secondary hypogonadism who needs his testosterone back to normal but also cares about fertility or avoiding long-term TRT drawbacks. In such patients, enclomiphene can improve symptoms of low T (fatigue, low libido, depression) with the peace of mind that their fertility is preserved. Its oral route and lack of controlled substance status make it convenient, though patients should be monitored regularly to ensure optimal outcomes. Typically, one would start at 12.5 mg daily and adjust to 25 mg if needed, checking testosterone and estradiol in a month. If the patient responds well, therapy can be continued with periodic evaluation of testosterone levels, blood counts, and prostate health as standard for any hypogonadal therapy.
In conclusion, enclomiphene represents a paradigm of treating male hypogonadism by stimulating rather than substituting. The quality of evidence supporting its use is robust for biochemical outcomes and reasonably good for safety, though direct evidence of long-term clinical outcomes (such as improved fertility success or long-term symptom relief) is still limited. Given it is essentially the active component of an FDA-approved drug (clomiphene), many clinicians are comfortable with its use under appropriate oversight. Patients should be informed that enclomiphene is off-label and therapy should be supervised by a knowledgeable provider, typically with expertise in male reproductive endocrinology. When used judiciously, enclomiphene can safely restore normal testosterone levels, improve well-being, and avoid the compromises that come with traditional TRT in men who want to remain fertile. It fills an important niche in the management of hypogonadism, and ongoing clinical experience continues to refine its role. As always, individualized patient care and monitoring are key, and further research (or eventual approval of similar agents) will help solidify the place of enclomiphene in hypogonadism treatment algorithms.
References: The information in this review is derived from multiple sources including peer-reviewed journal articles and clinical trial data and recent observational studies, among others, as cited throughout the text. These sources provide a comprehensive evidence base underpinning enclomiphene’s pharmacology, efficacy, safety, and clinical considerations.
No Comments.